VIDEO ENHANCED Cytophage Technologies Reports Positive Outcome from Innovative Bacteriophage Therapy in Patient with Antibiotic-Resistant Infection
 |
|||||||||
 |
 |
 |
|||||||
Winnipeg, December 17, 2024 – TheNewswire – Cytophage Technologies Ltd. (“Cytophage” or “the Company”) (TSXV: CYTO; FSE: 70G) is pleased to announce a positive outcome from the first Canadian patient treated with its pioneering bacteriophage (phage) therapy. Six months after receiving the treatment, the patient shows of significant a recovery, with fully-healed wounds, reduced inflammatory markers, and significantly improved functionality. The groundbreaking treatment was administered earlier this year to a patient suffering from a severe, life-threatening prosthetic joint infection (PJI).
Building on the success of this case, Cytophage is working to launch additional trials. “This case is a turning point for phage therapy in Canada,” said Dr. Steven Theriault, CEO of Cytophage. “We are committed to helping more patients overcome difficult-to-treat infections and advancing this re-emerging technology by expanding our network of partners to assist more patients suffering with PJI.”
Click Image To View Full Size
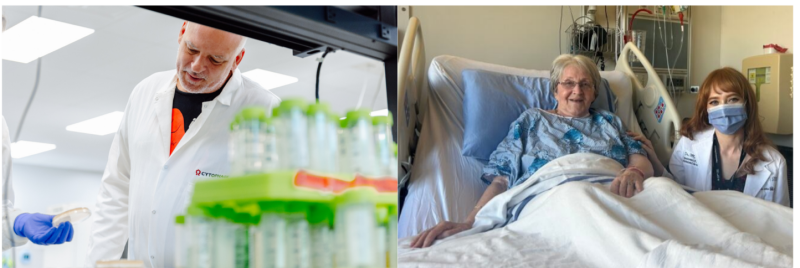
Left: Cytophage CEO in the company’s Canadian laboratories. Right: Patient receiving treatment in February, with Dr. Azad.
Cytophage Breakthrough Treatment Makes National Headlines
A Promising New Approach to a Life-Threatening Challenge
Last year, Cytophage was approached by Dr. Marisa Azad, a specialist in infectious diseases at The Ottawa Hospital, to help a patient battling an antibiotic-resistant infection associated with a joint replacement.
Facing a lack of effective treatment options, Dr. Azad collaborated with Cytophage to develop a unique phage therapy treatment plan, which was approved by Health Canada and delivered as a single-patient, open-label clinical trial (N of 1). Six months after receiving the therapy, the patient is now able to move with less pain, her wound has fully healed, and inflammation indicators in her blood have significantly decreased. Dr. Azad and her team continue to monitor the patient’s progress and plan to publish it as a case study in a peer-reviewed journal.
“This patient’s recovery has been transformative. We went from a situation where no antibiotic options were left, to seeing significant pain reduction, wound healing, and improved functionality,” says Dr. Azad. “This case is very promising – it opens the door to potentially help others with similar infections, and shows why further research and investment into phage therapy is urgently needed.”
Dr. Bradley Cook, Lead Scientist at Cytophage, says, “On behalf of the Cytophage laboratory team, we are incredibly proud and extend our heartfelt congratulations to the patient, Dr. Azad and her team. Witnessing the difference our work can make in people’s lives is humbling and inspiring.”
Addressing a Critical $2.6B Need
Joint replacements, such as hip and knee surgeries, are common in Canada, with over 130,000 procedures performed annually according to the Canadian Institute for Health Information (CIHI). These surgeries generally improve patients’ quality of life, but 1–2% are complicated by PJIs, a serious issue that can lead to amputation or even death if untreated. The economic burden of PJIs is substantial. Cytophage estimates that by 2030, the annual cost of PJIs to healthcare systems in the U.S. and Canada will exceed CAD $2.6 billion.
These complications exemplify the urgent problem of antimicrobial resistance, and the urgent need for innovative solutions like phage therapy to mitigate the rising economic and public-health burden. Cytophage believes that phage therapy could offer a cost-effective, less invasive treatment option for infections like PJIs, helping to reduce both their incidence and severity. By providing clinicians with an alternative that minimizes the need for complex revision surgeries, Cytophage’s approach has the potential to enable healthcare providers to reallocate substantial financial resources within their systems, ultimately benefiting more patients.
Next Steps
While Cytophage’s primary focus remains on developing bacteriophage solutions for animal health, the company is strategically expanding efforts to demonstrate the effectiveness of bacteriophages in treating challenging bacterial infections in human health. Cytophage is currently exploring collaboration opportunities with leading Canadian hospitals and clinics to showcase the potential of bacteriophages in safely and efficiently addressing orthopedic infections.
These partnerships with hospitals and clinics will enable Cytophage to identify and triage PJI patients in a cost-effective manner, providing an innovative treatment option for patients who have limited alternatives within the healthcare system. By working closely with these partners, Cytophage aims to build a robust dataset that will support clinical trial options as the company seeks to address a projected CAD$2.6-billion PJI market.
Dr. Marisa Azad is an Assistant Professor in Infectious Diseases at the University of Ottawa and an Associate Scientist at the Ottawa Hospital Research Institute. Her research focuses on innovative treatments and diagnostics for infections related to joint replacements. Dr. Azad and Cytophage are now working toward a multi-center trial to further explore the benefits of phage therapy for orthopedic infections.
About Cytophage Technologies
Cytophage Technologies (TSXV:CYTO / FSE: 70G) is a pioneering biotechnology company dedicated to bacteriophage research, product development and commercialization. Bacteriophages are viruses that only infect and kill bacteria. These natural killers of bacteria can overcome all cellular or organism-level defences. They are nature’s version of antibiotics.
Cytophage is improving bacteria’s natural predators with environmental as well as genetic modifications to bring safe and toxin free killer solutions to large addressable markets with an initial focus on animal health which offers a fast-track to near-term revenue. As the only bacteriophage manufacturer in Canada and powered by a large library of strains, Cytophage is committed to addressing the global challenge of antibiotic resistance (AMR).The WHO predicts that AMR will be the leading cause of human mortality by 2050. Many countries have already banned or limited preventative antibiotic use in animal production including 27 EU countries, US, Canada, Brazil, Bangladesh, India and Mexico. In addition to that, consumers all over the world are demanding organic and antibiotic-free products.
Cytophage is using a de-risked and patented technology to advance innovative and cost competitive products that harness the power of bacteriophages to combat bacterial infections affecting human health, animal health, and food security. The overall goal of Cytophage is to replace antibiotics down the road.
For more information, please visit www.cytophage.com and subscribe to our Investor Alerts: https://cytophage.com/subscribe.
For further information, please contact:
Heather Medwick
Chief Operating Officer
Tel.: +1 431 388 8873
Email: [email protected]
For media inquiries, please contact:
Simon Bredin ([email protected])
Cautionary Statement on Forward-Looking Information
This news release contains “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Any statement that involves discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Cytophage to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These risks include but are not limited to, the risks associated with the bacteriophage industry, such as operational risks in development or capital expenditures, the uncertainty of extensive regulatory approval requirements, government regulations, protection of intellectual property, product liability and rapid technological advancements. Forward-looking statements contained herein are made as of the date of this press release, and Cytophage disclaims, other than as required by law, any obligation to update any forward-looking statements whether as a result of new information, results, future events, circumstances, or if management’s estimates or opinions should change, or otherwise. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, the reader is cautioned not to place undue reliance on forward-looking statements.
Neither the TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this news release.
Copyright (c) 2024 TheNewswire – All rights reserved.





